How HBGVs are derived
Data and people
The HBGVs are derived by experts using appropriate data, e.g. scientific articles and reports of experimental studies. These studies can refer to studies with experimental animals such as mice and rats, but also with human volunteers and epidemiological studies. The experts usually work together in scientific panels in international organizations such as JECFA and EFSA. Although more rare, it is also possible that HBGVs are derived only by national experts, or even by one expert, e.g. when the HBGV is urgently needed.
Data analysis
The experts take the available data, and start with selecting relevant data, eliminating others. This process is based on expert opinion; there are no fixed rules or procedures how to weight the different data. Consequently there can be differences of opionion between the experts about the final result. After the selection of data, the experts have to define a dose that is considered the lowest effect dose in the studies. Here the type of observed effect and the dose-response of that effect is most important.
Effect levels
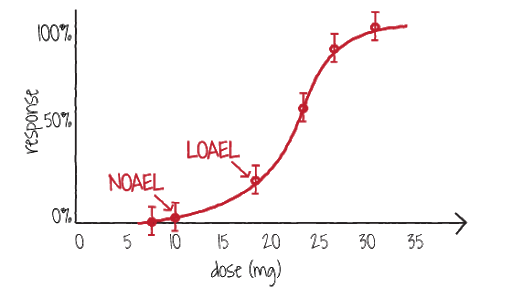 There are various terms for that effect dose; most used is the NOAEL. This acronym comes from "No Observed Adverse Effect Level". It should be the highest level in the experimental study without effect (response). This dose should show a response that is statistically not different from that of a control group. The effect should be reported as an adverse effect, i.e. injurious for the animal or human in the study. The effect should be Observed, meaning that it was actually seen in the study, e.g. a liver damage, or an increased or decreased level of an hormone or enzyme, or alike. Derived terms are the Lowest Observed Adverse Effect Level (LOAEL), or Marginal Observed Adverse Effect Level (MOAEL). Sometimes an effect is reported without the term Adverse (e.g. NOEL for a No Observed Effect Level).
There are various terms for that effect dose; most used is the NOAEL. This acronym comes from "No Observed Adverse Effect Level". It should be the highest level in the experimental study without effect (response). This dose should show a response that is statistically not different from that of a control group. The effect should be reported as an adverse effect, i.e. injurious for the animal or human in the study. The effect should be Observed, meaning that it was actually seen in the study, e.g. a liver damage, or an increased or decreased level of an hormone or enzyme, or alike. Derived terms are the Lowest Observed Adverse Effect Level (LOAEL), or Marginal Observed Adverse Effect Level (MOAEL). Sometimes an effect is reported without the term Adverse (e.g. NOEL for a No Observed Effect Level).
Setting the HBGV
The experts take an effect level, preferably the NOAEL. This is the basis for a HBGV like the ADI or TDI. The HBGV is now derived from the chosen effect level using safety factors (SF). These safety factors are actually extrapolation factors that take care of differences between the effect level and a "safe" exposure level for consumers. Most SFs are numbers of 10, and the common ADIs or TDIs are the NOAEL divided by 100 (i.e. 10 x 10). Additional safety factors of 10 can be used when using a LOAEL in stead of a NOAEL. Sometimes also SFs of 3 are being used, for example to extrapolate a MOAEL to a NOAEL. The SFs used to derive a HBGV are based on expert judgement of the panel members. There are guidelines for deriving SFs, but the underlying scientific basis for the weighing factors used as SFs is extremely limited. So the choise of SFs is a point of discussion between experts for many HBGVs, leading to the fact that different panels come with different HBGVs although using the same scientific data ena effect dose.
Summary
Based on the approach of setting an ADI it can be concluded that HBGVs are derived from effect levels selected from experimental studies, lowered by various safety factors of 3, or 10. The factors are there to extrapolate from the factual levels downto acceptable human exposure. Hereby it is assumed that the safety (i.e. extrapolation) factors are sufficient in all cases to protect human health from adverse effects as seen in the experimetal studies.
Both the selection of the relevant effect and the SF are based on expert judgement of one or more toxicological experts, leading to possible different outcomes between various groups of experts (e.g between EFSA and JECFA).